c2h2 lewis structure|c2h4 lewis structure : Cebu A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Ethyne or Acetylene).For the C2H2 structure use the periodic table to find the total . Daylight Saving: New Zealand Daylight Time (NZDT) is a daylight saving/summer time zone, however during winter some places adjust time for one hour back and observe New Zealand Standard Time (NZST). End: New Zealand Daylight Time (NZDT) has ended on Sunday, April 7, 2024 at 3:00 am local time and clocks were set one hour back to .
PH0 · c2h6o lewis structure
PH1 · c2h4 lewis structure
PH2 · c2h2 strukturformel
PH3 · c2h2 lewis formel
PH4 · c2h2 estructura de lewis
PH5 · c2h2 domain
PH6 · acetylene chemistry
PH7 · Iba pa
We would like to show you a description here but the site won’t allow us.
c2h2 lewis structure*******A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Ethyne or Acetylene).For the C2H2 structure use the periodic table to find the total .
In the Lewis structure of C2H2 structure there are a total of 10 valence electrons. C2H2 is also called Acetylene (Ethyne). ---- Steps to Write Lewis Structure for compounds like C2H2.c2h4 lewis structure Learn how to draw the Lewis structure of C2H2, a gaseous alkyne hydrocarbon, and understand its bond formation, geometry, hybridization and bond angle. See the video, diagrams and examples .Lewis Structure for C2H2 (Ethyne) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it .You can see the lewis structure of C 2 H 2 in above figure and you can see it is a simple structure. Now, we are going to draw that C 2 H 4 lewis structure step by step. Steps of drawing the lewis structure of C 2 H 2. .Learn how to draw the Lewis structure for C2H2, also known as ethyne or acetylene, a hydrocarbon compound with two triple bonds and two hydrogen atoms. Find out . Learn how to draw the stable Lewis structure of acetylene (C2H2) with five steps and examples. Find out the valence electrons, lone pairs, formal charges, and octet rule of C2H2.
Learn how to draw the C2H2 Lewis structure and its geometry using Lewis dot diagrams, and how to predict the polarity, bond angles, hybridization, and electron geometry .
With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms. Remember that Hydrogen (H) atoms .c2h2 lewis structureThe chemical formula of ethyne is C2H2. It consists of two carbon atoms and two hydrogen atoms. Ethyne is a colorless gas with a distinct odor and is highly flammable. The C2H2 Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. II. I quickly take you through how to draw the Lewis Structure of CHCH (Acetylene or ethyne). I also go over hybridization, shape, sigma, pi bonding and bond ang.A quick explanation of the molecular geometry of C2H2 including a description of the C2H2 bond angles. Note, the Hydrogen atoms (H) should not have lone pair.
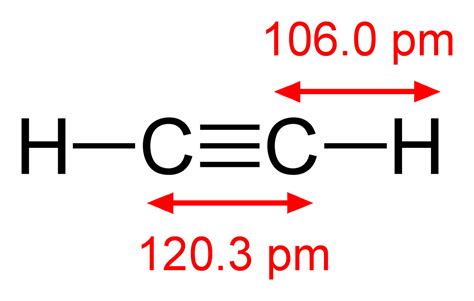
C2H2 lewis structure has a triple bond between the two Carbon atoms (C) and a single bond between the Carbon atom (C) and Hydrogen atom (H). If you haven’t understood anything from the above image of C2H2 lewis structure, then just stick with me and you will get the detailed step by step explanation on drawing a lewis structure of . Hey Guys,In this video we are going to learn about the Lewis structure of C2H2. It is a chemical formula for Ethyne or Acetylene.To understand the Lewis stru. Learn the steps to draw the Lewis Structure of C2H2 (ethyne or acetylene) in just 1 minute.📌You can draw any lewis structures by following the simple steps .Bonding in Ethane. In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3-hybridized, meaning that both have four bonds arranged with tetrahedral geometry.The carbon-carbon bond, with a bond length of 1.54 Å, is formed by overlap of one sp 3 orbital from each of the carbons, .C2H2 is a linear molecule in form of geometry and the Lewis structure of C2H2 shows that the carbon atom has four valence electrons, while the hydrogen atom has one valence electron as it is an s-block element. Name of Molecule. Acetylene or ethyne. Chemical Formula. C2H2.
La structure Lewis C2H2 a une triple liaison entre les deux atomes de carbone (C) et une liaison simple entre l’atome de carbone (C) et l’atome d’hydrogène (H). Si vous n’avez rien compris de l’image ci-dessus de la structure de Lewis de C2H2, alors restez avec moi et vous obtiendrez l’explication détaillée étape par étape sur .For the molecule acetylene (C2H2):a) Draw the Lewis structure from its constituent atoms.b) Predict the bond angle around one of the central carbon atoms.c) .Ethylene (commonly knows as ethene), CH 2 CH 2, is the simplest molecule which contains a carbon carbon double bond. The Lewis structure of ethylene indicates that there are one carbon-carbon double bond and four carbon-hydrogen single bonds. Experimentally, the four carbon-hydrogen bonds in the ethylene molecule have been shown to be identical. The total number of valence electrons in the acetylene or ethyne (C2H2) Lewis dot structure is 10. The molecular geometry or shape of C 2 H 2 is identical to its ideal electron pair geometry i.e., linear. The .

Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to .c2h2 lewis structure c2h4 lewis structure Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to . Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and .C2H2 is a tetratomic molecule made up of two distinct atoms that link in equal amounts. Furthermore, carbon bonds to carbon, giving acetylene a linear structure and a 180° bond angle. C2H2 Lewis Structure. The Lewis structure of C2H2 aids in the comprehension of the molecule’s shape.Lewis Dot of Ethyne (Acetylene) C 2 H 2. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Acetylene is an unsaturated hydrocarbon with a triple bond.Drawing the Lewis Structure for C 2 H 2 - Ethyne or Acetylene. Viewing Notes: With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms.; Remember that Hydrogen (H) atoms always go on the outside of a Lewis Structure. Note that Hydrogen only needs two valence electrons .
C2H2 Geometry and Hybridization. Because the hydrogens are always in terminal positions, the carbons must be connected, and therefore, we can draw a preliminary skeletal structure to start with: There are 2×4 + 2×1 = 10 valence, and 6 have been used to make 3 covalent bonds. Therefore, the remaining 4 go to the carbon atoms as a lone pair on .
Pure Taboo - Nothing To Lose. Niets te verliezen. sex dvd van Pure Taboo met Kit Mercer, Scarlett Mae, Lucky Fate, Alex Jett, Jimmy Broadway. Sex dvds. Ga naar zoeken Ga naar hoofdinhoud. lekker winkelen zonder zorgen. Gratis verzending vanaf 20,- .
c2h2 lewis structure|c2h4 lewis structure